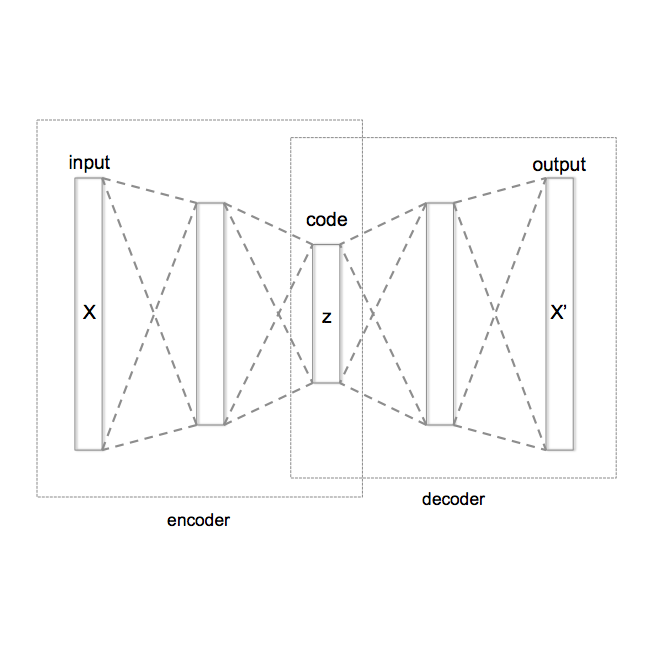
Head-based signals such as EEG, EMG, EOG, and ECG collected by wearable systems will play a pivotal role in clinical diagnosis, monitoring, and treatment of important brain disorder diseases. However, the real-time transmission of the significant corpus physiological signals over extended periods consumes substantial power and time, limiting the viability of battery-dependent physiological monitoring wearables. This paper presents a novel deep-learning framework employing a variational autoencoder (VAE) for physiological signal compression to reduce wearables' computational complexity and energy consumption. Our approach achieves an impressive compression ratio of 1:293 specifically for spectrogram data, surpassing state-of-the-art compression techniques such as JPEG2000, H.264, Direct Cosine Transform (DCT), and Huffman Encoding, which do not excel in handling physiological signals. We validate the efficacy of the compressed algorithms using collected physiological signals from real patients in the Hospital and deploy the solution on commonly used embedded AI chips (i.e., ARM Cortex V8 and Jetson Nano). The proposed framework achieves a 91% seizure detection accuracy using XGBoost, confirming the approach's reliability, practicality, and scalability.
相關內容
Class imbalance is a pervasive issue in the field of disease classification from medical images. It is necessary to balance out the class distribution while training a model for decent results. However, in the case of rare medical diseases, images from affected patients are much harder to come by compared to images from non-affected patients, resulting in unwanted class imbalance. Various processes of tackling class imbalance issues have been explored so far, each having its fair share of drawbacks. In this research, we propose an outlier detection based binary medical image classification technique which can handle even the most extreme case of class imbalance. We have utilized a dataset of malaria parasitized and uninfected cells. An autoencoder model titled AnoMalNet is trained with only the uninfected cell images at the beginning and then used to classify both the affected and non-affected cell images by thresholding a loss value. We have achieved an accuracy, precision, recall, and F1 score of 98.49%, 97.07%, 100%, and 98.52% respectively, performing better than large deep learning models and other published works. As our proposed approach can provide competitive results without needing the disease-positive samples during training, it should prove to be useful in binary disease classification on imbalanced datasets.
Clinical calculators play a vital role in healthcare by offering accurate evidence-based predictions for various purposes such as prognosis. Nevertheless, their widespread utilization is frequently hindered by usability challenges, poor dissemination, and restricted functionality. Augmenting large language models with extensive collections of clinical calculators presents an opportunity to overcome these obstacles and improve workflow efficiency, but the scalability of the manual curation process poses a significant challenge. In response, we introduce AgentMD, a novel language agent capable of curating and applying clinical calculators across various clinical contexts. Using the published literature, AgentMD has automatically curated a collection of 2,164 diverse clinical calculators with executable functions and structured documentation, collectively named RiskCalcs. Manual evaluations show that RiskCalcs tools achieve an accuracy of over 80% on three quality metrics. At inference time, AgentMD can automatically select and apply the relevant RiskCalcs tools given any patient description. On the newly established RiskQA benchmark, AgentMD significantly outperforms chain-of-thought prompting with GPT-4 (87.7% vs. 40.9% in accuracy). Additionally, we also applied AgentMD to real-world clinical notes for analyzing both population-level and risk-level patient characteristics. In summary, our study illustrates the utility of language agents augmented with clinical calculators for healthcare analytics and patient care.

Optical coherence tomography (OCT) is a non-invasive, micrometer-scale imaging modality that has become a clinical standard in ophthalmology. By raster-scanning the retina, sequential cross-sectional image slices are acquired to generate volumetric data. In-vivo imaging suffers from discontinuities between slices that show up as motion and illumination artifacts. We present a new illumination model that exploits continuity in orthogonally raster-scanned volume data. Our novel spatiotemporal parametrization adheres to illumination continuity both temporally, along the imaged slices, as well as spatially, in the transverse directions. Yet, our formulation does not make inter-slice assumptions, which could have discontinuities. This is the first optimization of a 3D inverse model in an image reconstruction context in OCT. Evaluation in 68 volumes from eyes with pathology showed reduction of illumination artifacts in 88\% of the data, and only 6\% showed moderate residual illumination artifacts. The method enables the use of forward-warped motion corrected data, which is more accurate, and enables supersampling and advanced 3D image reconstruction in OCT.
Survival risk stratification is an important step in clinical decision making for breast cancer management. We propose a novel deep learning approach for this purpose by integrating histopathological imaging, genetic and clinical data. It employs vision transformers, specifically the MaxViT model, for image feature extraction, and self-attention to capture intricate image relationships at the patient level. A dual cross-attention mechanism fuses these features with genetic data, while clinical data is incorporated at the final layer to enhance predictive accuracy. Experiments on the public TCGA-BRCA dataset show that our model, trained using the negative log likelihood loss function, can achieve superior performance with a mean C-index of 0.64, surpassing existing methods. This advancement facilitates tailored treatment strategies, potentially leading to improved patient outcomes.
Fingerprint recognition stands as a pivotal component of biometric technology, with diverse applications from identity verification to advanced search tools. In this paper, we propose a unique method for deriving robust fingerprint representations by leveraging enhancement-based pre-training. Building on the achievements of U-Net-based fingerprint enhancement, our method employs a specialized encoder to derive representations from fingerprint images in a self-supervised manner. We further refine these representations, aiming to enhance the verification capabilities. Our experimental results, tested on publicly available fingerprint datasets, reveal a marked improvement in verification performance against established self-supervised training techniques. Our findings not only highlight the effectiveness of our method but also pave the way for potential advancements. Crucially, our research indicates that it is feasible to extract meaningful fingerprint representations from degraded images without relying on enhanced samples.

The use of hyperspectral imaging for medical applications is becoming more common in recent years. One of the main obstacles that researchers find when developing hyperspectral algorithms for medical applications is the lack of specific, publicly available, and hyperspectral medical data. The work described in this paper was developed within the framework of the European project HELICoiD (HypErspectraL Imaging Cancer Detection), which had as a main goal the application of hyperspectral imaging to the delineation of brain tumors in real-time during neurosurgical operations. In this paper, the methodology followed to generate the first hyperspectral database of in-vivo human brain tissues is presented. Data was acquired employing a customized hyperspectral acquisition system capable of capturing information in the Visual and Near InfraRed (VNIR) range from 400 to 1000 nm. Repeatability was assessed for the cases where two images of the same scene were captured consecutively. The analysis reveals that the system works more efficiently in the spectral range between 450 and 900 nm. A total of 36 hyperspectral images from 22 different patients were obtained. From these data, more than 300 000 spectral signatures were labeled employing a semi-automatic methodology based on the spectral angle mapper algorithm. Four different classes were defined: normal tissue, tumor tissue, blood vessel, and background elements. All the hyperspectral data has been made available in a public repository.
Malaria is a major health issue worldwide, and its diagnosis requires scalable solutions that can work effectively with low-cost microscopes (LCM). Deep learning-based methods have shown success in computer-aided diagnosis from microscopic images. However, these methods need annotated images that show cells affected by malaria parasites and their life stages. Annotating images from LCM significantly increases the burden on medical experts compared to annotating images from high-cost microscopes (HCM). For this reason, a practical solution would be trained on HCM images which should generalize well on LCM images during testing. While earlier methods adopted a multi-stage learning process, they did not offer an end-to-end approach. In this work, we present an end-to-end learning framework, named CodaMal (Contrastive Domain Adpation for Malaria). In order to bridge the gap between HCM (training) and LCM (testing), we propose a domain adaptive contrastive loss. It reduces the domain shift by promoting similarity between the representations of HCM and its corresponding LCM image, without imposing an additional annotation burden. In addition, the training objective includes object detection objectives with carefully designed augmentations, ensuring the accurate detection of malaria parasites. On the publicly available large-scale M5-dataset, our proposed method shows a significant improvement of 16% over the state-of-the-art methods in terms of the mean average precision metric (mAP), provides 21x speed up during inference, and requires only half learnable parameters than the prior methods. Our code is publicly available.
Image registration is a critical component in the applications of various medical image analyses. In recent years, there has been a tremendous surge in the development of deep learning (DL)-based medical image registration models. This paper provides a comprehensive review of medical image registration. Firstly, a discussion is provided for supervised registration categories, for example, fully supervised, dual supervised, and weakly supervised registration. Next, similarity-based as well as generative adversarial network (GAN)-based registration are presented as part of unsupervised registration. Deep iterative registration is then described with emphasis on deep similarity-based and reinforcement learning-based registration. Moreover, the application areas of medical image registration are reviewed. This review focuses on monomodal and multimodal registration and associated imaging, for instance, X-ray, CT scan, ultrasound, and MRI. The existing challenges are highlighted in this review, where it is shown that a major challenge is the absence of a training dataset with known transformations. Finally, a discussion is provided on the promising future research areas in the field of DL-based medical image registration.
It has been shown that deep neural networks are prone to overfitting on biased training data. Towards addressing this issue, meta-learning employs a meta model for correcting the training bias. Despite the promising performances, super slow training is currently the bottleneck in the meta learning approaches. In this paper, we introduce a novel Faster Meta Update Strategy (FaMUS) to replace the most expensive step in the meta gradient computation with a faster layer-wise approximation. We empirically find that FaMUS yields not only a reasonably accurate but also a low-variance approximation of the meta gradient. We conduct extensive experiments to verify the proposed method on two tasks. We show our method is able to save two-thirds of the training time while still maintaining the comparable or achieving even better generalization performance. In particular, our method achieves the state-of-the-art performance on both synthetic and realistic noisy labels, and obtains promising performance on long-tailed recognition on standard benchmarks.
Applying artificial intelligence techniques in medical imaging is one of the most promising areas in medicine. However, most of the recent success in this area highly relies on large amounts of carefully annotated data, whereas annotating medical images is a costly process. In this paper, we propose a novel method, called FocalMix, which, to the best of our knowledge, is the first to leverage recent advances in semi-supervised learning (SSL) for 3D medical image detection. We conducted extensive experiments on two widely used datasets for lung nodule detection, LUNA16 and NLST. Results show that our proposed SSL methods can achieve a substantial improvement of up to 17.3% over state-of-the-art supervised learning approaches with 400 unlabeled CT scans.