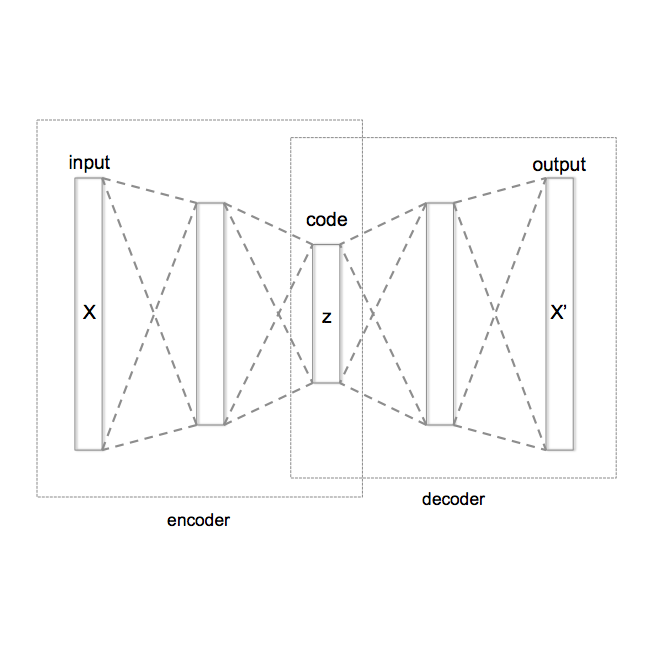
Head-based signals such as EEG, EMG, EOG, and ECG collected by wearable systems will play a pivotal role in clinical diagnosis, monitoring, and treatment of important brain disorder diseases. However, the real-time transmission of the significant corpus physiological signals over extended periods consumes substantial power and time, limiting the viability of battery-dependent physiological monitoring wearables. This paper presents a novel deep-learning framework employing a variational autoencoder (VAE) for physiological signal compression to reduce wearables' computational complexity and energy consumption. Our approach achieves an impressive compression ratio of 1:293 specifically for spectrogram data, surpassing state-of-the-art compression techniques such as JPEG2000, H.264, Direct Cosine Transform (DCT), and Huffman Encoding, which do not excel in handling physiological signals. We validate the efficacy of the compressed algorithms using collected physiological signals from real patients in the Hospital and deploy the solution on commonly used embedded AI chips (i.e., ARM Cortex V8 and Jetson Nano). The proposed framework achieves a 91{\%} seizure detection accuracy using XGBoost, confirming the approach's reliability, practicality, and scalability.
相關內容
Early detection of myocardial infarction (MI), a critical condition arising from coronary artery disease (CAD), is vital to prevent further myocardial damage. This study introduces a novel method for early MI detection using a one-class classification (OCC) algorithm in echocardiography. Our study overcomes the challenge of limited echocardiography data availability by adopting a novel approach based on Multi-modal Subspace Support Vector Data Description. The proposed technique involves a specialized MI detection framework employing multi-view echocardiography incorporating a composite kernel in the non-linear projection trick, fusing Gaussian and Laplacian sigmoid functions. Additionally, we enhance the update strategy of the projection matrices by adapting maximization for both or one of the modalities in the optimization process. Our method boosts MI detection capability by efficiently transforming features extracted from echocardiography data into an optimized lower-dimensional subspace. The OCC model trained specifically on target class instances from the comprehensive HMC-QU dataset that includes multiple echocardiography views indicates a marked improvement in MI detection accuracy. Our findings reveal that our proposed multi-view approach achieves a geometric mean of 71.24\%, signifying a substantial advancement in echocardiography-based MI diagnosis and offering more precise and efficient diagnostic tools.
Resilience against stragglers is a critical element of prediction serving systems, tasked with executing inferences on input data for a pre-trained machine-learning model. In this paper, we propose NeRCC, as a general straggler-resistant framework for approximate coded computing. NeRCC includes three layers: (1) encoding regression and sampling, which generates coded data points, as a combination of original data points, (2) computing, in which a cluster of workers run inference on the coded data points, (3) decoding regression and sampling, which approximately recovers the predictions of the original data points from the available predictions on the coded data points. We argue that the overall objective of the framework reveals an underlying interconnection between two regression models in the encoding and decoding layers. We propose a solution to the nested regressions problem by summarizing their dependence on two regularization terms that are jointly optimized. Our extensive experiments on different datasets and various machine learning models, including LeNet5, RepVGG, and Vision Transformer (ViT), demonstrate that NeRCC accurately approximates the original predictions in a wide range of stragglers, outperforming the state-of-the-art by up to 23%.
Due to the lack of quality annotation in medical imaging community, semi-supervised learning methods are highly valued in image semantic segmentation tasks. In this paper, an advanced consistency-aware pseudo-label-based self-ensembling approach is presented to fully utilize the power of Vision Transformer(ViT) and Convolutional Neural Network(CNN) in semi-supervised learning. Our proposed framework consists of a feature-learning module which is enhanced by ViT and CNN mutually, and a guidance module which is robust for consistency-aware purposes. The pseudo labels are inferred and utilized recurrently and separately by views of CNN and ViT in the feature-learning module to expand the data set and are beneficial to each other. Meanwhile, a perturbation scheme is designed for the feature-learning module, and averaging network weight is utilized to develop the guidance module. By doing so, the framework combines the feature-learning strength of CNN and ViT, strengthens the performance via dual-view co-training, and enables consistency-aware supervision in a semi-supervised manner. A topological exploration of all alternative supervision modes with CNN and ViT are detailed validated, demonstrating the most promising performance and specific setting of our method on semi-supervised medical image segmentation tasks. Experimental results show that the proposed method achieves state-of-the-art performance on a public benchmark data set with a variety of metrics. The code is publicly available.
Multi-agent pathfinding (MAPF) is a critical field in many large-scale robotic applications, often being the fundamental step in multi-agent systems. The increasing complexity of MAPF in complex and crowded environments, however, critically diminishes the effectiveness of existing solutions. In contrast to other studies that have either presented a general overview of the recent advancements in MAPF or extensively reviewed Deep Reinforcement Learning (DRL) within multi-agent system settings independently, our work presented in this review paper focuses on highlighting the integration of DRL-based approaches in MAPF. Moreover, we aim to bridge the current gap in evaluating MAPF solutions by addressing the lack of unified evaluation metrics and providing comprehensive clarification on these metrics. Finally, our paper discusses the potential of model-based DRL as a promising future direction and provides its required foundational understanding to address current challenges in MAPF. Our objective is to assist readers in gaining insight into the current research direction, providing unified metrics for comparing different MAPF algorithms and expanding their knowledge of model-based DRL to address the existing challenges in MAPF.
Objective: Ultrasound (US) examination has unique advantages in diagnosing carpal tunnel syndrome (CTS) while identifying the median nerve (MN) and diagnosing CTS depends heavily on the expertise of examiners. To alleviate this problem, we aimed to develop a one-stop automated CTS diagnosis system (OSA-CTSD) and evaluate its effectiveness as a computer-aided diagnostic tool. Methods: We combined real-time MN delineation, accurate biometric measurements, and explainable CTS diagnosis into a unified framework, called OSA-CTSD. We collected a total of 32,301 static images from US videos of 90 normal wrists and 40 CTS wrists for evaluation using a simplified scanning protocol. Results: The proposed model showed better segmentation and measurement performance than competing methods, reporting that HD95 score of 7.21px, ASSD score of 2.64px, Dice score of 85.78%, and IoU score of 76.00%, respectively. In the reader study, it demonstrated comparable performance with the average performance of the experienced in classifying the CTS, while outperformed that of the inexperienced radiologists in terms of classification metrics (e.g., accuracy score of 3.59% higher and F1 score of 5.85% higher). Conclusion: The OSA-CTSD demonstrated promising diagnostic performance with the advantages of real-time, automation, and clinical interpretability. The application of such a tool can not only reduce reliance on the expertise of examiners, but also can help to promote the future standardization of the CTS diagnosis process, benefiting both patients and radiologists.
Modern compilers, such as LLVM, are complex pieces of software. Due to their complexity, manual testing is unlikely to suffice, yet formal verification is difficult to scale. End-to-end fuzzing can be used, but it has difficulties in achieving high coverage of some components of LLVM. In this paper, we implement IRFuzzer to investigate the effectiveness of specialized fuzzing of the LLVM compiler backend. We focus on two approaches to improve the fuzzer: guaranteed input validity using constrained mutations and improved feedback quality. The mutator in IRFuzzer is capable of generating a wide range of LLVM IR inputs, including structured control flow, vector types, and function definitions. The system instruments coding patterns in the compiler to monitor the execution status of instruction selection. The instrumentation not only provides a new coverage feedback called matcher table coverage, but also provides an architecture specific guidance to the mutator. We show that IRFuzzer is more effective than existing fuzzers by fuzzing on 29 mature LLVM backend targets. In the process, we reported 74 confirmed new bugs in LLVM upstream, out of which 49 have been fixed, five have been back ported to LLVM 15, showing that specialized fuzzing provides useful and actionable insights to LLVM developers.
Feature attribution methods (FAs), such as gradients and attention, are widely employed approaches to derive the importance of all input features to the model predictions. Existing work in natural language processing has mostly focused on developing and testing FAs for encoder-only language models (LMs) in classification tasks. However, it is unknown if it is faithful to use these FAs for decoder-only models on text generation, due to the inherent differences between model architectures and task settings respectively. Moreover, previous work has demonstrated that there is no `one-wins-all' FA across models and tasks. This makes the selection of a FA computationally expensive for large LMs since input importance derivation often requires multiple forward and backward passes including gradient computations that might be prohibitive even with access to large compute. To address these issues, we present a model-agnostic FA for generative LMs called Recursive Attribution Generator (ReAGent). Our method updates the token importance distribution in a recursive manner. For each update, we compute the difference in the probability distribution over the vocabulary for predicting the next token between using the original input and using a modified version where a part of the input is replaced with RoBERTa predictions. Our intuition is that replacing an important token in the context should have resulted in a larger change in the model's confidence in predicting the token than replacing an unimportant token. Our method can be universally applied to any generative LM without accessing internal model weights or additional training and fine-tuning, as most other FAs require. We extensively compare the faithfulness of ReAGent with seven popular FAs across six decoder-only LMs of various sizes. The results show that our method consistently provides more faithful token importance distributions.
In recent advancements in medical image analysis, Convolutional Neural Networks (CNN) and Vision Transformers (ViT) have set significant benchmarks. While the former excels in capturing local features through its convolution operations, the latter achieves remarkable global context understanding by leveraging self-attention mechanisms. However, both architectures exhibit limitations in efficiently modeling long-range dependencies within medical images, which is a critical aspect for precise segmentation. Inspired by the Mamba architecture, known for its proficiency in handling long sequences and global contextual information with enhanced computational efficiency as a State Space Model (SSM), we propose Mamba-UNet, a novel architecture that synergizes the U-Net in medical image segmentation with Mamba's capability. Mamba-UNet adopts a pure Visual Mamba (VMamba)-based encoder-decoder structure, infused with skip connections to preserve spatial information across different scales of the network. This design facilitates a comprehensive feature learning process, capturing intricate details and broader semantic contexts within medical images. We introduce a novel integration mechanism within the VMamba blocks to ensure seamless connectivity and information flow between the encoder and decoder paths, enhancing the segmentation performance. We conducted experiments on publicly available MRI cardiac multi-structures segmentation dataset. The results show that Mamba-UNet outperforms UNet, Swin-UNet in medical image segmentation under the same hyper-parameter setting. The source code and baseline implementations are available.
Image registration is a critical component in the applications of various medical image analyses. In recent years, there has been a tremendous surge in the development of deep learning (DL)-based medical image registration models. This paper provides a comprehensive review of medical image registration. Firstly, a discussion is provided for supervised registration categories, for example, fully supervised, dual supervised, and weakly supervised registration. Next, similarity-based as well as generative adversarial network (GAN)-based registration are presented as part of unsupervised registration. Deep iterative registration is then described with emphasis on deep similarity-based and reinforcement learning-based registration. Moreover, the application areas of medical image registration are reviewed. This review focuses on monomodal and multimodal registration and associated imaging, for instance, X-ray, CT scan, ultrasound, and MRI. The existing challenges are highlighted in this review, where it is shown that a major challenge is the absence of a training dataset with known transformations. Finally, a discussion is provided on the promising future research areas in the field of DL-based medical image registration.
Applying artificial intelligence techniques in medical imaging is one of the most promising areas in medicine. However, most of the recent success in this area highly relies on large amounts of carefully annotated data, whereas annotating medical images is a costly process. In this paper, we propose a novel method, called FocalMix, which, to the best of our knowledge, is the first to leverage recent advances in semi-supervised learning (SSL) for 3D medical image detection. We conducted extensive experiments on two widely used datasets for lung nodule detection, LUNA16 and NLST. Results show that our proposed SSL methods can achieve a substantial improvement of up to 17.3% over state-of-the-art supervised learning approaches with 400 unlabeled CT scans.