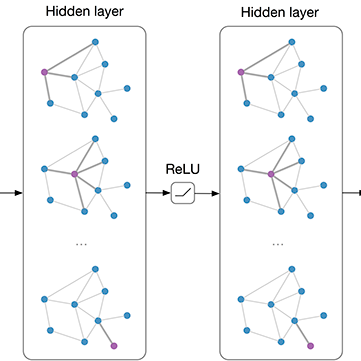
Focal cortical dysplasia (FCD) is a leading cause of drug-resistant focal epilepsy, which can be cured by surgery. These lesions are extremely subtle and often missed even by expert neuroradiologists. "Ground truth" manual lesion masks are therefore expensive, limited and have large inter-rater variability. Existing FCD detection methods are limited by high numbers of false positive predictions, primarily due to vertex- or patch-based approaches that lack whole-brain context. Here, we propose to approach the problem as semantic segmentation using graph convolutional networks (GCN), which allows our model to learn spatial relationships between brain regions. To address the specific challenges of FCD identification, our proposed model includes an auxiliary loss to predict distance from the lesion to reduce false positives and a weak supervision classification loss to facilitate learning from uncertain lesion masks. On a multi-centre dataset of 1015 participants with surface-based features and manual lesion masks from structural MRI data, the proposed GCN achieved an AUC of 0.74, a significant improvement against a previously used vertex-wise multi-layer perceptron (MLP) classifier (AUC 0.64). With sensitivity thresholded at 67%, the GCN had a specificity of 71% in comparison to 49% when using the MLP. This improvement in specificity is vital for clinical integration of lesion-detection tools into the radiological workflow, through increasing clinical confidence in the use of AI radiological adjuncts and reducing the number of areas requiring expert review.
相關內容
Active learning algorithms have become increasingly popular for training models with limited data. However, selecting data for annotation remains a challenging problem due to the limited information available on unseen data. To address this issue, we propose EdgeAL, which utilizes the edge information of unseen images as {\it a priori} information for measuring uncertainty. The uncertainty is quantified by analyzing the divergence and entropy in model predictions across edges. This measure is then used to select superpixels for annotation. We demonstrate the effectiveness of EdgeAL on multi-class Optical Coherence Tomography (OCT) segmentation tasks, where we achieved a 99% dice score while reducing the annotation label cost to 12%, 2.3%, and 3%, respectively, on three publicly available datasets (Duke, AROI, and UMN). The source code is available at \url{//github.com/Mak-Ta-Reque/EdgeAL}
Graph-based neural network models are gaining traction in the field of representation learning due to their ability to uncover latent topological relationships between entities that are otherwise challenging to identify. These models have been employed across a diverse range of domains, encompassing drug discovery, protein interactions, semantic segmentation, and fluid dynamics research. In this study, we investigate the potential of Graph Neural Networks (GNNs) for medical image classification. We introduce a novel model that combines GNNs and edge convolution, leveraging the interconnectedness of RGB channel feature values to strongly represent connections between crucial graph nodes. Our proposed model not only performs on par with state-of-the-art Deep Neural Networks (DNNs) but does so with 1000 times fewer parameters, resulting in reduced training time and data requirements. We compare our Graph Convolutional Neural Network (GCNN) to pre-trained DNNs for classifying MedMNIST dataset classes, revealing promising prospects for GNNs in medical image analysis. Our results also encourage further exploration of advanced graph-based models such as Graph Attention Networks (GAT) and Graph Auto-Encoders in the medical imaging domain. The proposed model yields more reliable, interpretable, and accurate outcomes for tasks like semantic segmentation and image classification compared to simpler GCNNs
Graph anomaly detection (GAD) is a vital task since even a few anomalies can pose huge threats to benign users. Recent semi-supervised GAD methods, which can effectively leverage the available labels as prior knowledge, have achieved superior performances than unsupervised methods. In practice, people usually need to identify anomalies on new (sub)graphs to secure their business, but they may lack labels to train an effective detection model. One natural idea is to directly adopt a trained GAD model to the new (sub)graph for testing. However, we find that existing semi-supervised GAD methods suffer from poor generalization issue, i.e., well-trained models could not perform well on an unseen area (i.e., not accessible in training) of the same graph. It may cause great troubles. In this paper, we base on the phenomenon and propose a general and novel research problem of generalized graph anomaly detection that aims to effectively identify anomalies on both the training-domain graph and unseen testing graph to eliminate potential dangers. Nevertheless, it is a challenging task since only limited labels are available, and the normal background may differ between training and testing data. Accordingly, we propose a data augmentation method named \textit{AugAN} (\uline{Aug}mentation for \uline{A}nomaly and \uline{N}ormal distributions) to enrich training data and boost the generalizability of GAD models. Experiments verify the effectiveness of our method in improving model generalizability.
Medical image segmentation is a challenging task, particularly due to inter- and intra-observer variability, even between medical experts. In this paper, we propose a novel model, called Probabilistic Inter-Observer and iNtra-Observer variation NetwOrk (Pionono). It captures the labeling behavior of each rater with a multidimensional probability distribution and integrates this information with the feature maps of the image to produce probabilistic segmentation predictions. The model is optimized by variational inference and can be trained end-to-end. It outperforms state-of-the-art models such as STAPLE, Probabilistic U-Net, and models based on confusion matrices. Additionally, Pionono predicts multiple coherent segmentation maps that mimic the rater's expert opinion, which provides additional valuable information for the diagnostic process. Experiments on real-world cancer segmentation datasets demonstrate the high accuracy and efficiency of Pionono, making it a powerful tool for medical image analysis.
Data augmentation has been widely used to improve generalizability of machine learning models. However, comparatively little work studies data augmentation for graphs. This is largely due to the complex, non-Euclidean structure of graphs, which limits possible manipulation operations. Augmentation operations commonly used in vision and language have no analogs for graphs. Our work studies graph data augmentation for graph neural networks (GNNs) in the context of improving semi-supervised node-classification. We discuss practical and theoretical motivations, considerations and strategies for graph data augmentation. Our work shows that neural edge predictors can effectively encode class-homophilic structure to promote intra-class edges and demote inter-class edges in given graph structure, and our main contribution introduces the GAug graph data augmentation framework, which leverages these insights to improve performance in GNN-based node classification via edge prediction. Extensive experiments on multiple benchmarks show that augmentation via GAug improves performance across GNN architectures and datasets.
Breast cancer remains a global challenge, causing over 1 million deaths globally in 2018. To achieve earlier breast cancer detection, screening x-ray mammography is recommended by health organizations worldwide and has been estimated to decrease breast cancer mortality by 20-40%. Nevertheless, significant false positive and false negative rates, as well as high interpretation costs, leave opportunities for improving quality and access. To address these limitations, there has been much recent interest in applying deep learning to mammography; however, obtaining large amounts of annotated data poses a challenge for training deep learning models for this purpose, as does ensuring generalization beyond the populations represented in the training dataset. Here, we present an annotation-efficient deep learning approach that 1) achieves state-of-the-art performance in mammogram classification, 2) successfully extends to digital breast tomosynthesis (DBT; "3D mammography"), 3) detects cancers in clinically-negative prior mammograms of cancer patients, 4) generalizes well to a population with low screening rates, and 5) outperforms five-out-of-five full-time breast imaging specialists by improving absolute sensitivity by an average of 14%. Our results demonstrate promise towards software that can improve the accuracy of and access to screening mammography worldwide.
Video anomaly detection under weak labels is formulated as a typical multiple-instance learning problem in previous works. In this paper, we provide a new perspective, i.e., a supervised learning task under noisy labels. In such a viewpoint, as long as cleaning away label noise, we can directly apply fully supervised action classifiers to weakly supervised anomaly detection, and take maximum advantage of these well-developed classifiers. For this purpose, we devise a graph convolutional network to correct noisy labels. Based upon feature similarity and temporal consistency, our network propagates supervisory signals from high-confidence snippets to low-confidence ones. In this manner, the network is capable of providing cleaned supervision for action classifiers. During the test phase, we only need to obtain snippet-wise predictions from the action classifier without any extra post-processing. Extensive experiments on 3 datasets at different scales with 2 types of action classifiers demonstrate the efficacy of our method. Remarkably, we obtain the frame-level AUC score of 82.12% on UCF-Crime.

Recent advances in 3D fully convolutional networks (FCN) have made it feasible to produce dense voxel-wise predictions of volumetric images. In this work, we show that a multi-class 3D FCN trained on manually labeled CT scans of several anatomical structures (ranging from the large organs to thin vessels) can achieve competitive segmentation results, while avoiding the need for handcrafting features or training class-specific models. To this end, we propose a two-stage, coarse-to-fine approach that will first use a 3D FCN to roughly define a candidate region, which will then be used as input to a second 3D FCN. This reduces the number of voxels the second FCN has to classify to ~10% and allows it to focus on more detailed segmentation of the organs and vessels. We utilize training and validation sets consisting of 331 clinical CT images and test our models on a completely unseen data collection acquired at a different hospital that includes 150 CT scans, targeting three anatomical organs (liver, spleen, and pancreas). In challenging organs such as the pancreas, our cascaded approach improves the mean Dice score from 68.5 to 82.2%, achieving the highest reported average score on this dataset. We compare with a 2D FCN method on a separate dataset of 240 CT scans with 18 classes and achieve a significantly higher performance in small organs and vessels. Furthermore, we explore fine-tuning our models to different datasets. Our experiments illustrate the promise and robustness of current 3D FCN based semantic segmentation of medical images, achieving state-of-the-art results. Our code and trained models are available for download: //github.com/holgerroth/3Dunet_abdomen_cascade.
High spectral dimensionality and the shortage of annotations make hyperspectral image (HSI) classification a challenging problem. Recent studies suggest that convolutional neural networks can learn discriminative spatial features, which play a paramount role in HSI interpretation. However, most of these methods ignore the distinctive spectral-spatial characteristic of hyperspectral data. In addition, a large amount of unlabeled data remains an unexploited gold mine for efficient data use. Therefore, we proposed an integration of generative adversarial networks (GANs) and probabilistic graphical models for HSI classification. Specifically, we used a spectral-spatial generator and a discriminator to identify land cover categories of hyperspectral cubes. Moreover, to take advantage of a large amount of unlabeled data, we adopted a conditional random field to refine the preliminary classification results generated by GANs. Experimental results obtained using two commonly studied datasets demonstrate that the proposed framework achieved encouraging classification accuracy using a small number of data for training.
Image segmentation is considered to be one of the critical tasks in hyperspectral remote sensing image processing. Recently, convolutional neural network (CNN) has established itself as a powerful model in segmentation and classification by demonstrating excellent performances. The use of a graphical model such as a conditional random field (CRF) contributes further in capturing contextual information and thus improving the segmentation performance. In this paper, we propose a method to segment hyperspectral images by considering both spectral and spatial information via a combined framework consisting of CNN and CRF. We use multiple spectral cubes to learn deep features using CNN, and then formulate deep CRF with CNN-based unary and pairwise potential functions to effectively extract the semantic correlations between patches consisting of three-dimensional data cubes. Effective piecewise training is applied in order to avoid the computationally expensive iterative CRF inference. Furthermore, we introduce a deep deconvolution network that improves the segmentation masks. We also introduce a new dataset and experimented our proposed method on it along with several widely adopted benchmark datasets to evaluate the effectiveness of our method. By comparing our results with those from several state-of-the-art models, we show the promising potential of our method.